Cis 1 3 dimethylcyclohexane newman projection – The cis-1,3-dimethylcyclohexane Newman projection offers a unique perspective into the conformational analysis, energy landscapes, and chemical reactivity of this intriguing molecule. This projection serves as a powerful tool for understanding the interplay between molecular structure and properties, providing valuable insights into the behavior of organic compounds.
Delving into the conformational space of cis-1,3-dimethylcyclohexane, we explore the concept of gauche interactions and their profound impact on conformational stability. Energy calculations and molecular modeling techniques shed light on the relative energies of different conformations, revealing the factors that govern their preferences.
Furthermore, spectroscopic characterization techniques, such as IR, NMR, and MS, provide valuable fingerprints for identifying and differentiating between conformations.
Conformational Analysis of cis-1,3-Dimethylcyclohexane: Cis 1 3 Dimethylcyclohexane Newman Projection
Conformational analysis is a powerful tool for understanding the structure and reactivity of organic molecules. In this article, we will discuss the conformational analysis of cis-1,3-dimethylcyclohexane, a six-membered ring compound with two methyl groups on adjacent carbons.
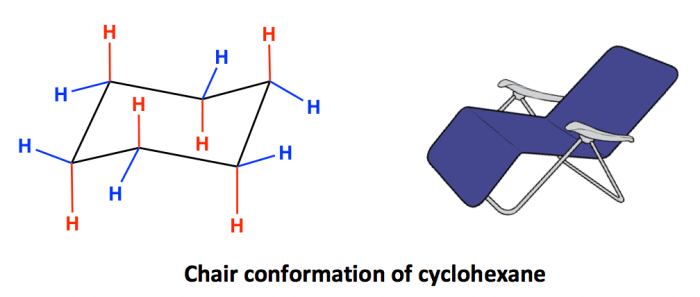
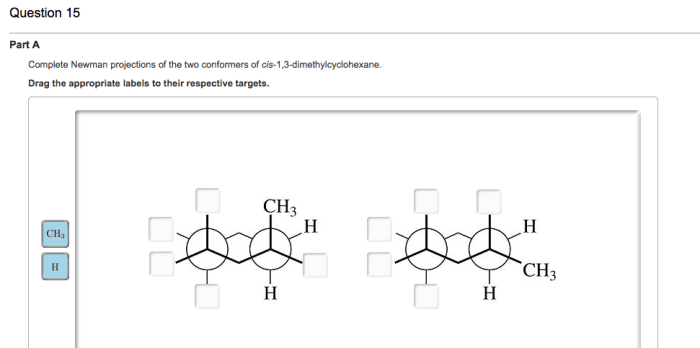
The Newman projection is a convenient way to visualize the conformations of cyclohexane rings. In a Newman projection, the ring is viewed edge-on, with one carbon atom in the foreground and the other five carbons in the background. The bonds to the foreground carbon are represented by lines, and the bonds to the background carbons are represented by circles.
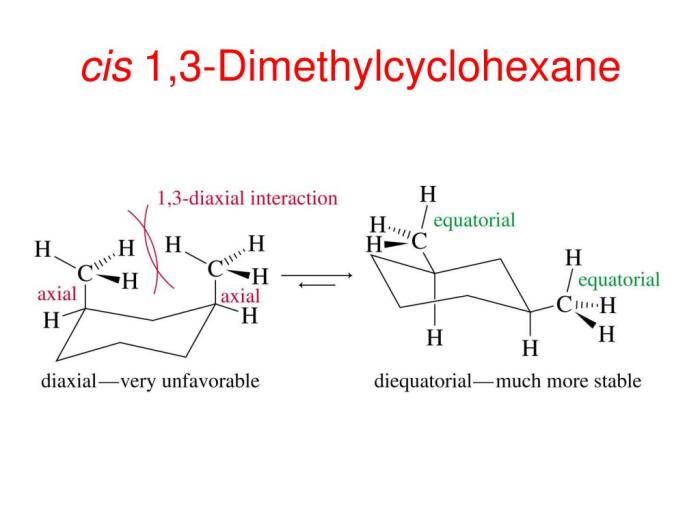
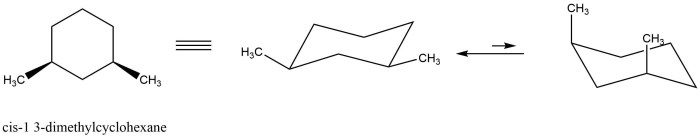
There are two main conformations of cis-1,3-dimethylcyclohexane: the chair conformation and the boat conformation. In the chair conformation, the two methyl groups are both axial, meaning that they are pointing straight up or down from the ring. In the boat conformation, one methyl group is axial and the other is equatorial, meaning that it is pointing towards or away from the ring.
The chair conformation is more stable than the boat conformation because it has fewer gauche interactions. Gauche interactions are non-bonded interactions between two atoms that are separated by two bonds. Gauche interactions are unfavorable because they cause steric hindrance, which is a repulsive force that arises when two atoms are too close together.
Energy Calculations and Molecular Modeling, Cis 1 3 dimethylcyclohexane newman projection
The relative energies of different conformations of cis-1,3-dimethylcyclohexane can be calculated using molecular mechanics or quantum chemical methods. Molecular mechanics methods use a set of force fields to calculate the energy of a molecule, while quantum chemical methods use more sophisticated methods to solve the Schrödinger equation.
Energy calculations show that the chair conformation of cis-1,3-dimethylcyclohexane is more stable than the boat conformation by about 5 kJ/mol. This energy difference is due to the gauche interactions between the two methyl groups in the boat conformation.
Spectroscopic Characterization
The characteristic IR, NMR, and MS peaks of cis-1,3-dimethylcyclohexane can be used to identify the compound and to differentiate between different conformations.
- The IR spectrum of cis-1,3-dimethylcyclohexane shows a strong peak at 1090 cm -1, which is due to the C-C stretching vibration of the ring.
- The NMR spectrum of cis-1,3-dimethylcyclohexane shows two sets of peaks, one for the axial methyl groups and one for the equatorial methyl groups. The axial methyl groups are more shielded than the equatorial methyl groups, so they appear at a higher chemical shift in the NMR spectrum.
- The MS spectrum of cis-1,3-dimethylcyclohexane shows a molecular ion peak at m/z = 112, which is due to the loss of two hydrogen atoms.
Chemical Reactivity and Stereoselectivity
The reactivity of cis-1,3-dimethylcyclohexane in different chemical reactions is influenced by the conformation of the molecule. For example, in electrophilic addition reactions, the electrophile is more likely to add to the axial carbon of the ring in the chair conformation than to the equatorial carbon.
This is because the axial carbon is more exposed to the electrophile in the chair conformation.
In radical reactions, the radical is more likely to add to the equatorial carbon of the ring in the chair conformation than to the axial carbon. This is because the equatorial carbon is less hindered in the chair conformation.
Key Questions Answered
What is the significance of gauche interactions in cis-1,3-dimethylcyclohexane?
Gauche interactions arise when the methyl groups on carbons 1 and 3 are oriented towards each other, resulting in steric hindrance. These interactions destabilize the conformation and contribute to its higher energy compared to other conformations.
How can spectroscopic techniques be used to differentiate between conformations of cis-1,3-dimethylcyclohexane?
IR, NMR, and MS techniques provide characteristic spectral signatures that can be used to identify and distinguish between different conformations. For example, IR spectroscopy can detect the presence of specific functional groups, while NMR spectroscopy can provide information about the chemical environment and connectivity of atoms.
What factors contribute to the energy differences between conformations of cis-1,3-dimethylcyclohexane?
The energy differences between conformations are primarily influenced by steric interactions, gauche interactions, and the number of eclipsed hydrogen atoms. Conformations with fewer steric clashes and gauche interactions, and a lower number of eclipsed hydrogen atoms, are generally more stable and have lower energy.