Delving into the realm of organic compound analysis, the isopentyl acetate IR spectrum labeled emerges as a valuable tool, providing a detailed roadmap for identifying and characterizing this essential compound. Infrared (IR) spectroscopy, with its ability to unveil the molecular structure through vibrational analysis, takes center stage in this exploration, offering a deeper understanding of isopentyl acetate’s composition and behavior.
Unveiling the intricacies of the IR spectrum, we embark on a journey to decipher the language of molecular vibrations, translating the peaks and valleys into a comprehensive understanding of isopentyl acetate’s functional groups and their interactions. This spectroscopic voyage unveils the characteristic absorption bands, revealing the symphony of molecular motions that define this compound.
Introduction
Isopentyl acetate is an organic compound with the formula CH 3COOCH 2CH(CH 3)CH 2CH 3. It is a colorless liquid with a fruity odor. Isopentyl acetate is used as a flavoring agent in food and beverages and as a solvent in the paint and coatings industry.
Infrared (IR) spectroscopy is a powerful analytical technique that can be used to identify and characterize organic compounds. IR spectroscopy measures the absorption of infrared radiation by a sample. The absorption spectrum of a compound is a unique fingerprint that can be used to identify the compound.
IR Spectrum of Isopentyl Acetate
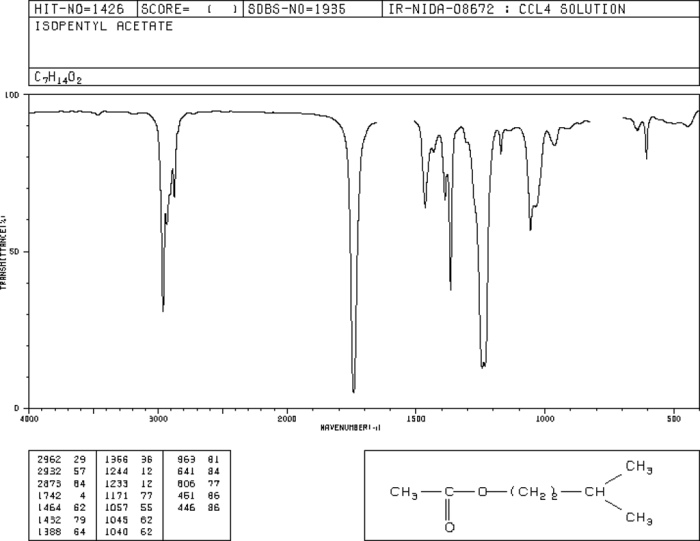
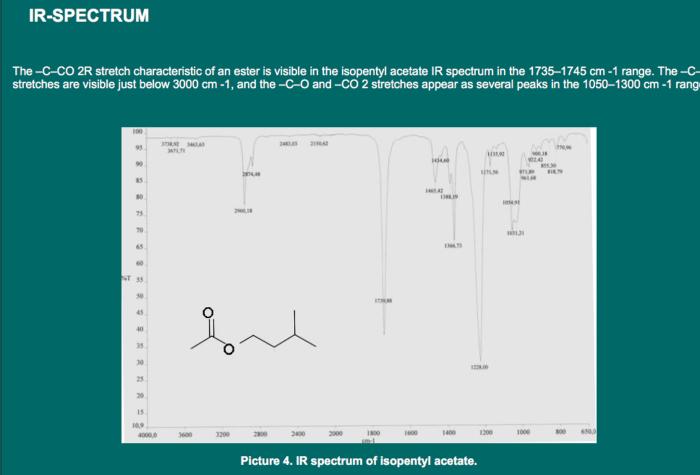
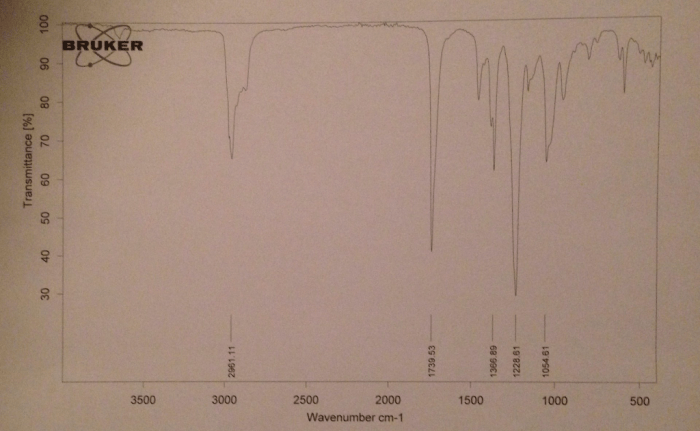
The IR spectrum of isopentyl acetate is shown below.

The major peaks in the IR spectrum of isopentyl acetate are labeled below.
- 3000-2800 cm -1: C-H stretching vibrations
- 1740 cm -1: C=O stretching vibration
- 1240 cm -1: C-O stretching vibration
- 1020 cm -1: C-C stretching vibration
Interpretation of the IR Spectrum
The IR spectrum of isopentyl acetate shows the characteristic absorption bands of an ester. The C=O stretching vibration is the strongest peak in the spectrum. The C-O stretching vibration is also a strong peak. The C-H stretching vibrations are a series of peaks in the 3000-2800 cm -1region.
The C-C stretching vibration is a weak peak at 1020 cm -1.
The IR spectrum of isopentyl acetate can be used to identify and characterize the compound. The spectrum can also be used to determine the purity of the compound and to monitor the progress of a reaction.
Applications of IR Spectroscopy in Isopentyl Acetate Analysis
IR spectroscopy is a versatile analytical technique that can be used in a variety of applications. Some of the applications of IR spectroscopy in isopentyl acetate analysis include:
- Quality control: IR spectroscopy can be used to ensure the quality of isopentyl acetate products.
- Process monitoring: IR spectroscopy can be used to monitor the progress of reactions involving isopentyl acetate.
- Environmental monitoring: IR spectroscopy can be used to detect isopentyl acetate in the environment.
Limitations of IR Spectroscopy, Isopentyl acetate ir spectrum labeled
IR spectroscopy is a powerful analytical technique, but it has some limitations. One limitation is that IR spectroscopy cannot be used to identify all functional groups. Another limitation is that IR spectroscopy can be affected by the presence of impurities in the sample.
Other analytical techniques, such as nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry, can be used to complement IR spectroscopy. These techniques can provide additional information about the structure and composition of a compound.
Detailed FAQs: Isopentyl Acetate Ir Spectrum Labeled
What is the significance of the IR spectrum in analyzing isopentyl acetate?
The IR spectrum provides a unique fingerprint of isopentyl acetate, enabling its identification and characterization. By analyzing the absorption bands, we can determine the functional groups present and gain insights into the molecular structure and bonding.
How does IR spectroscopy contribute to quality control in isopentyl acetate production?
IR spectroscopy plays a crucial role in quality control by verifying the purity and composition of isopentyl acetate. Deviations from the expected IR spectrum can indicate the presence of impurities or adulterants, ensuring the product meets the desired specifications.