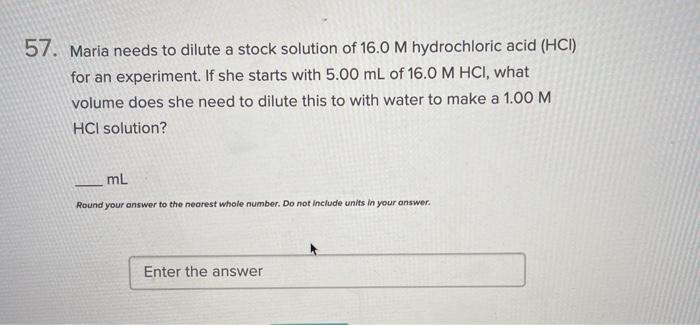
Maria needs to dilute the stock of 15.0, embarking on a scientific endeavor that involves the careful manipulation of solutions to achieve a desired concentration. This guide delves into the concept of stock dilution, exploring its intricacies and providing a step-by-step calculation for Maria’s specific task.
Moreover, the discussion encompasses the impact of dilution on stock properties, safety considerations, and practical applications.
Stock dilution is a fundamental technique in laboratory settings, enabling researchers to adjust the concentration of solutions to suit experimental requirements. By understanding the principles and calculations involved, scientists can ensure accurate and reproducible results in their experiments.
Stock Dilution Concept: Maria Needs To Dilute The Stock Of 15.0
Stock dilution is a technique used to reduce the concentration of a stock solution by adding a solvent, typically water. This process is commonly employed in laboratory settings to obtain solutions with lower concentrations for various experimental purposes.
An example of stock dilution is preparing a 10 mM stock solution from a 100 mM stock solution. By adding a specific volume of water to the 100 mM stock, the concentration can be diluted to 10 mM.
Diluting Stock Concentration
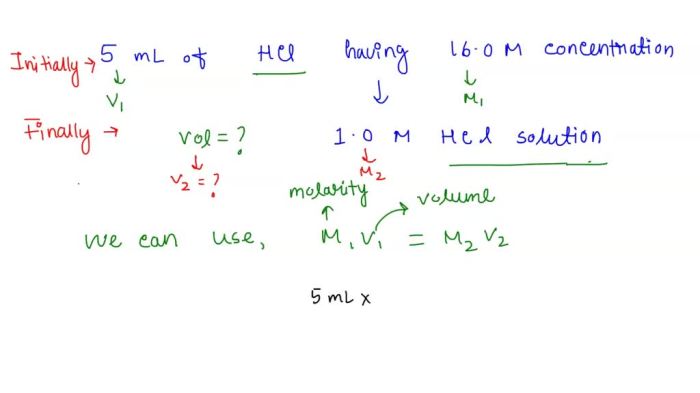
To dilute a stock solution, a specific volume of the stock solution is mixed with a calculated volume of the solvent. The formula for calculating the new stock concentration after dilution is:
C 1V 1= C 2V 2
- C 1is the initial stock concentration
- V 1is the initial stock volume
- C 2is the final stock concentration
- V 2is the final stock volume
Dilution Calculations for Maria’s Stock
Let’s say Maria needs to dilute her 15.0 mM stock solution to a concentration of 5.0 mM. She has 10 mL of the 15.0 mM stock solution.
| Parameter | Initial Value | Final Value |
|---|---|---|
| Initial Concentration (C1) | 15.0 mM | 5.0 mM |
| Initial Volume (V1) | 10 mL | V2 (to be calculated) |
Using the formula C 1V 1= C 2V 2, we can calculate the final volume (V 2):
15.0 mM – 10 mL = 5.0 mM – V 2
V 2= 30 mL
Therefore, Maria needs to add 20 mL of water to her 10 mL of 15.0 mM stock solution to obtain a final volume of 30 mL with a concentration of 5.0 mM.
Impact of Dilution on Stock Properties
Dilution affects the concentration, volume, and molarity of the stock solution. Concentration is inversely proportional to volume, meaning as the volume increases, the concentration decreases. Molarity is directly proportional to concentration, so as the concentration decreases, the molarity also decreases.
The relationship between these properties can be summarized as follows:
- Concentration (C) ∝ 1/Volume (V)
- Molarity (M) ∝ Concentration (C)
Applications of Stock Dilution
Stock dilution is commonly used in:
- Preparing solutions with lower concentrations for experiments
- Creating calibration curves for analytical techniques
- Extending the shelf life of stock solutions by reducing their concentration
Benefits of stock dilution include:
- Accurate preparation of solutions with specific concentrations
- Cost-effective use of stock solutions
Limitations of stock dilution include:
- Potential for errors in calculations or measurements
- Possibility of contamination if proper aseptic techniques are not followed
Safety Considerations
When diluting stock solutions, it is essential to take the following safety precautions:
- Wear appropriate personal protective equipment (PPE), including gloves and eye protection.
- Use a calibrated pipette or graduated cylinder to measure volumes accurately.
- Mix the stock solution and solvent thoroughly to ensure homogeneity.
- Dispose of diluted stock solutions properly according to laboratory protocols.
Potential hazards associated with stock dilution include:
- Exposure to hazardous chemicals
- Cuts or punctures from glassware
- Contamination of solutions
FAQ Resource
What is the purpose of stock dilution?
Stock dilution is used to adjust the concentration of a stock solution to a desired level, making it suitable for specific experimental requirements.
How do I calculate the new stock concentration after dilution?
To calculate the new stock concentration (C2), use the formula: C2 = C1 x V1 / V2, where C1 is the initial stock concentration, V1 is the initial stock volume, and V2 is the final stock volume.
What are the safety precautions to consider when diluting stock solutions?
Always wear appropriate personal protective equipment, such as gloves and eye protection. Handle concentrated stock solutions with care, and dispose of them properly according to laboratory regulations.