Lab activity kool aid concentration – The “Lab Activity: Kool-Aid Concentration” experiment delves into the fascinating realm of solution chemistry, inviting us to explore the concept of concentration and its practical applications. By harnessing the power of spectrophotometry, we embark on a journey to unravel the relationship between the concentration of Kool-Aid solutions and their light-absorbing properties, unveiling the secrets that lie within these vibrant concoctions.
In this engaging exploration, we will dissect the purpose and materials required for this captivating lab activity, ensuring safety precautions are meticulously observed. We will delve into the intricacies of solution concentration, unraveling the mysteries of varying Kool-Aid concentrations and the techniques for their precise preparation.
Armed with this foundational knowledge, we will venture into the realm of spectrophotometry, deciphering its fundamental principles and mastering the art of measuring Kool-Aid solution concentrations using this sophisticated analytical tool.
Lab Activity: Kool-Aid Concentration

The purpose of this lab activity is to determine the concentration of a Kool-Aid solution by measuring its absorbance at a specific wavelength using a spectrophotometer.
The materials needed for this activity include:
- Kool-Aid powder
- Water
- Graduated cylinder
- Beaker
- Spectrophotometer
- Cuvette
Safety Precautions
The following safety precautions should be taken when performing this activity:
- Wear gloves and safety goggles.
- Do not ingest or inhale the Kool-Aid powder.
- Dispose of the Kool-Aid solution properly.
Kool-Aid Solutions: Lab Activity Kool Aid Concentration
In chemistry, a solution is a homogeneous mixture of two or more substances. The solute is the substance that is dissolved in the solvent. In the case of Kool-Aid, the solute is the powdered drink mix and the solvent is water.
The concentration of a solution is a measure of the amount of solute that is dissolved in a given amount of solvent. The concentration of a Kool-Aid solution can be expressed in several ways, including mass per volume (mg/mL), moles per liter (mol/L), or parts per million (ppm).
Preparing Kool-Aid Solutions of Varying Concentrations
To prepare a Kool-Aid solution of a specific concentration, you will need to know the following information:
- The desired concentration of the solution
- The mass of the Kool-Aid powder
- The volume of the solution
Once you have this information, you can use the following formula to calculate the mass of Kool-Aid powder that you need to add to the water:
Mass of Kool-Aid powder = Concentration of solution x Volume of solution
For example, if you want to prepare a 10% (m/v) Kool-Aid solution, you would need to add 10 grams of Kool-Aid powder to 100 mL of water.
Spectrophotometer Analysis
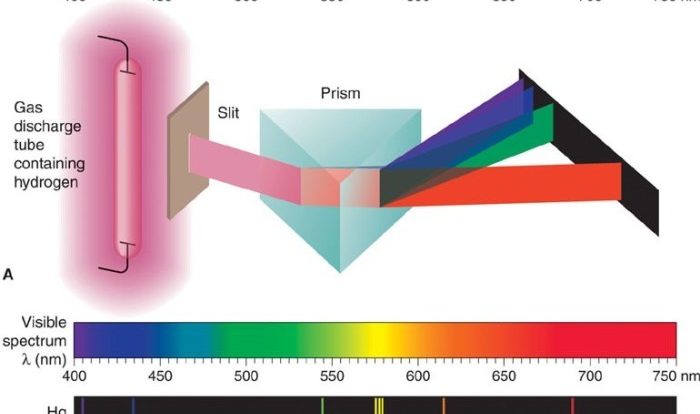
Spectrophotometry is a technique that measures the amount of light absorbed by a substance at specific wavelengths. This absorption is directly proportional to the concentration of the substance in the sample.
In the context of Kool-Aid solutions, a spectrophotometer can be used to measure the concentration of the dye in the solution. The dye in Kool-Aid absorbs light at a specific wavelength, which can be determined using a spectrophotometer. By measuring the absorbance of the solution at this wavelength, the concentration of the dye can be determined.
Step-by-Step Procedure for Spectrophotometer Analysis, Lab activity kool aid concentration
- Prepare a series of Kool-Aid solutions with known concentrations.
- Calibrate the spectrophotometer using a blank solution (a solution that does not contain any dye).
- Measure the absorbance of each Kool-Aid solution at the wavelength of maximum absorption.
- Plot a calibration curve of absorbance versus concentration.
- Use the calibration curve to determine the concentration of the unknown Kool-Aid solution.
Data Analysis and Interpretation
After collecting the spectrophotometer data, we need to analyze and interpret it to determine the relationship between the concentration of the Kool-Aid solutions and their absorbance.
Creating a Table to Organize Data
The first step is to create a table to organize the data. The table should include the following columns:
- Concentration (M)
- Absorbance (Abs)
Plotting a Graph of Concentration versus Absorbance
Once the data is organized, we can plot a graph of concentration versus absorbance. The graph should have concentration on the x-axis and absorbance on the y-axis.
Relationship between Concentration and Absorbance
The graph of concentration versus absorbance should show a linear relationship. This means that as the concentration of the Kool-Aid solution increases, the absorbance also increases. The slope of the line represents the molar absorptivity of the Kool-Aid solution.
The molar absorptivity is a constant that is characteristic of the Kool-Aid solution. It is a measure of how strongly the Kool-Aid solution absorbs light at a particular wavelength.
Applications of Kool-Aid Concentration
Understanding Kool-Aid concentration has numerous practical applications in various fields, including food and beverage production, chemistry, and environmental science.
Concentration plays a crucial role in determining the taste, sweetness, and color of food and beverages. In the food industry, controlling Kool-Aid concentration is essential for ensuring consistent product quality and meeting consumer preferences.
Chemistry
- In chemistry, concentration is used to determine the amount of a substance present in a solution. This information is critical for various chemical reactions and analytical techniques, such as titrations and spectrophotometry.
- Kool-Aid solutions can be used as a model system to study the principles of concentration and its impact on chemical reactions.
Environmental Science
- In environmental science, understanding Kool-Aid concentration can help monitor and control water quality. By measuring the concentration of pollutants in water samples, scientists can assess the level of contamination and take appropriate measures to mitigate its impact.
li>Kool-Aid solutions can be used to simulate water samples with varying concentrations of pollutants, allowing researchers to study the effectiveness of different water treatment methods.
Q&A
What is the purpose of this lab activity?
The purpose of this lab activity is to investigate the concept of concentration in solutions and to learn how to use a spectrophotometer to measure the concentration of a solution.
What materials are needed for this activity?
The materials needed for this activity include Kool-Aid powder, water, graduated cylinders, pipettes, spectrophotometer, cuvettes, and a light source.
What safety precautions should be taken?
The safety precautions that should be taken include wearing gloves and eye protection, and avoiding contact with the spectrophotometer lamp.